Your Hand sanitizer fda product code images are available in this site. Hand sanitizer fda product code are a topic that is being searched for and liked by netizens today. You can Download the Hand sanitizer fda product code files here. Get all royalty-free photos and vectors.
If you’re looking for hand sanitizer fda product code images information related to the hand sanitizer fda product code interest, you have pay a visit to the right blog. Our website frequently provides you with suggestions for seeing the maximum quality video and image content, please kindly hunt and find more informative video articles and graphics that match your interests.
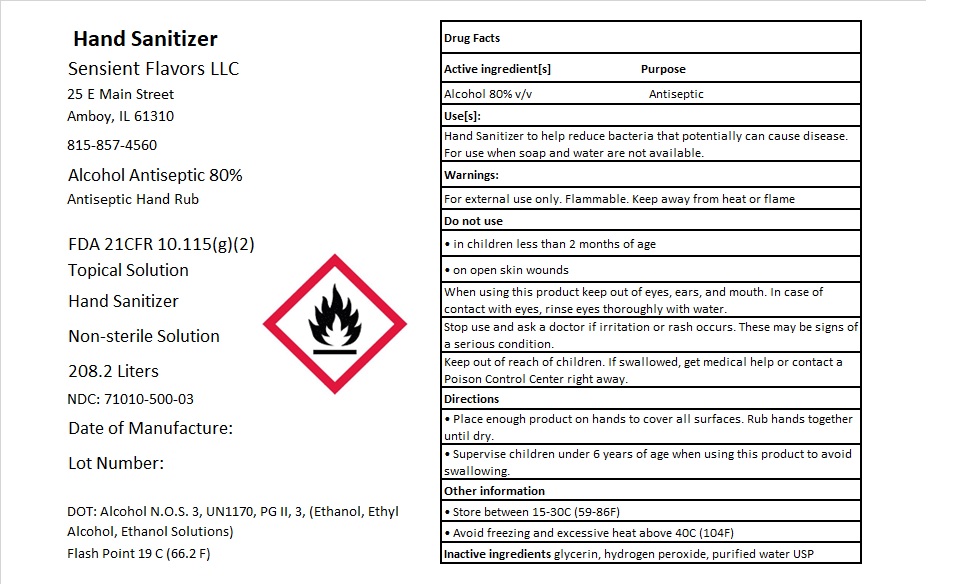
Hand Sanitizer Fda Product Code. If the product code is valid the name of the product will appear on the next screen. The FDAs guidance documents apply only to handrub products prepared using the United States Pharmacopoeia or Food Chemical Codex grade ingredients specifically described in the guidance consistent with World Health Organization recommendations from Coronavirus COVID-19 Update. This designation means that youll need to follow plenty of regulations to keep your hands clean of any label violations. If you have a product code and want to know if it is still a valid code or if you are not sure what product it represents you can enter the code in the appropriate fields.
 Scentsational Soaps Candles Recalls Scented Hand Sanitizers Due To Presence Of Methanol Benzene And Acetaldehyde Wgn Radio 720 Chicago S Very Own From wgnradio.com
Scentsational Soaps Candles Recalls Scented Hand Sanitizers Due To Presence Of Methanol Benzene And Acetaldehyde Wgn Radio 720 Chicago S Very Own From wgnradio.com
OTC Drug products that do meet such FDA conditions must follow the FDAs standard drug requirements for approval OTC monograph new drug application-NDA etc. If the product code is valid the name of the product will appear on the next screen. Continue reading FDA hand sanitizer registration FDA requirements for hand sanitizer or FDA approval for hand sanitizer. FDA Regulations for Hand Sanitizers. Each listed drug is assigned a National Drug Code NDC. FDA provides guidance on production of alcohol-based hand sanitizer to help boost supply protect.
For importing the hand sanitizer the Customs filerbroker must declare the companys registration number to FDA when filing the entry.
To view primary and secondary products for this product code. For importing the hand sanitizer the Customs filerbroker must declare the companys registration number to FDA when filing the entry. There may be secondary products with this product code. If you are planning to market hand sanitizers or any form of antiseptic products we can help you in fulfilling FDA. FDA Hand Sanitizer Registration Approval - I3CGLOBAL Hand Sanitizer FDA Registration Approval Hand Sanitizer is considered as the counter drug OTC as per US FDA regulation. Antiseptic Hand sanitizers are OTC drugs which require FDA establishment registration Drug listing and NDC Labeler code also known as NDC Number.
 Source: amazon.com
Source: amazon.com
The Application returns the primary product for the product code entered. If the product code is valid the name of the product will appear on the next screen. The Application returns the primary product for the product code entered. Option 4 - Verify Product Code. Hand Sanitizer - Active Ingredients benzalkonium chloride Alcohol ethyl alcohol or ethanol 60 to 95 percent and isopropyl alcohol 70 to 913 percent are still under FDA OTC Drug review and are eligible for marketing manufacturers of hand sanitizers with these ingredients continue with FDA drug establishment registration renewal and drug listing update every year.
 Source: pasco1999.en.made-in-china.com
Source: pasco1999.en.made-in-china.com
Amid the novel coronavirus outbreak COVID-19 we are getting many questions about FDA requirements for hand sanitizers and other questions related to FDA regulations for hand sanitizers. If the product code is valid the name of the product will appear on the next screen. Continue reading FDA hand sanitizer registration FDA requirements for hand sanitizer or FDA approval for hand sanitizer. If Soap and Water Are Not Available. Cosmereg reviews ingredients and labeling requirements following OTC monograph requirements and suggest to manufacturersbrand owner of Hand Sanitizer if the product is in line with the FDA hand sanitizer regulations before proceeding with the Registration.
 Source: globalsources.com
Source: globalsources.com
The Application returns the primary product for the product code entered. Companies that only import the hand sanitizers do not manufacture repack or relabel the product do not need to register with FDA. 4 Zeilen Active Ingredient in OTC Hand Sanitizer. FDA Regulations for Hand Sanitizers. By building upon the code portions you select the application will provide valid choices for each of the five components of the product code Industry Class Subclass PIC and Product.
 Source: farmhousefreshgoods.com
Source: farmhousefreshgoods.com
The Product Code Builder online toolapplication will guide you through an easy and user friendly selection process that will assist in locating and building a product code. Hand sanitizer with FDA. To view primary and secondary products for this product code. 25113 - Dodge 25113-444 - Instant Hand Sanitizer 25113-444-15. For importing the hand sanitizer the Customs filerbroker must declare the companys registration number to FDA when filing the entry.
 Source: pinterest.com
Source: pinterest.com
If you have a product code and want to know if it is still a valid code or if you are not sure what product it represents you can enter the code in the appropriate fields. Companies that only import the hand sanitizers do not manufacture repack or relabel the product do not need to register with FDA. If you are planning to market Alcohol or Benzalkonium based hand sanitizer you have to comply with FDA requirements for hand sanitizer listed below. After the product is submitted and get approved manufacturers and brand owners can export their products to the US. To view primary and secondary products for this product code.
 Source: fda.report
Source: fda.report
Continue reading FDA hand sanitizer registration FDA requirements for hand sanitizer or FDA approval for hand sanitizer. Hand Sanitizers and COVID-19. FDA Hand Sanitizer Labeling Requirements. So manufacturing import or distribution is permitted only after FDA Hand Sanitizer Registration and Listing. No FDA approved applications pursuant to section 505 of the Act 21 USC.
 Source: pinterest.com
Source: pinterest.com
Hand Sanitizer - Active Ingredients benzalkonium chloride Alcohol ethyl alcohol or ethanol 60 to 95 percent and isopropyl alcohol 70 to 913 percent are still under FDA OTC Drug review and are eligible for marketing manufacturers of hand sanitizers with these ingredients continue with FDA drug establishment registration renewal and drug listing update every year. For importing the hand sanitizer the Customs filerbroker must declare the companys registration number to FDA when filing the entry. If you have a product code and want to know if it is still a valid code or if you are not sure what product it represents you can enter the code in the appropriate fields. To view primary and secondary products for this product code. The most common active ingredient used in Hand sanitizers are ethyl alcohol or ethanol and Isopropyl alcohol complying with OTC Monograph not final part 333 A.
 Source: eworldtrade.com
Source: eworldtrade.com
The non-proprietary name is sometimes called the generic name. FDA Regulations for Hand Sanitizers. 4 Zeilen Active Ingredient in OTC Hand Sanitizer. The Application returns the primary product for the product code entered. Hand Sanitizer - Active Ingredients benzalkonium chloride Alcohol ethyl alcohol or ethanol 60 to 95 percent and isopropyl alcohol 70 to 913 percent are still under FDA OTC Drug review and are eligible for marketing manufacturers of hand sanitizers with these ingredients continue with FDA drug establishment registration renewal and drug listing update every year.
 Source: wgnradio.com
Source: wgnradio.com
If the product code is valid the name of the product will appear on the next screen. Cosmereg can assist you in complying with the FDAs Cosmetic Drug Establishment Registration request the labeler code and Drug Listing no matter the specific category. The Application returns the primary product for the product code entered. The generic name usually includes the active ingredients of the product. For importing the hand sanitizer the Customs filerbroker must declare the companys registration number to FDA when filing the entry.
 Source: medisouthstore.com
Source: medisouthstore.com
Registered drug establishments must list the drug eg. FDA approval for hand sanitizer FDA approval is not required for OTC hand sanitizers. Cosmereg can assist you in complying with the FDAs Cosmetic Drug Establishment Registration request the labeler code and Drug Listing no matter the specific category. The full process can take from 7 to 15 working days. If Soap and Water Are Not Available.
 Source: fda.report
Source: fda.report
There may be secondary products with this product code. 4 Zeilen Active Ingredient in OTC Hand Sanitizer. FDA provides guidance on production of alcohol-based hand sanitizer to help boost supply protect. After the product is submitted and get approved manufacturers and brand owners can export their products to the US. For importing the hand sanitizer the Customs filerbroker must declare the companys registration number to FDA when filing the entry.
 Source: besthopebath.en.made-in-china.com
Source: besthopebath.en.made-in-china.com
If you are planning to market hand sanitizers or any form of antiseptic products we can help you in fulfilling FDA. The FDAs guidance documents apply only to handrub products prepared using the United States Pharmacopoeia or Food Chemical Codex grade ingredients specifically described in the guidance consistent with World Health Organization recommendations from Coronavirus COVID-19 Update. FDA Hand Sanitizer Registration Approval - I3CGLOBAL Hand Sanitizer FDA Registration Approval Hand Sanitizer is considered as the counter drug OTC as per US FDA regulation. 4 Zeilen Active Ingredient in OTC Hand Sanitizer. The Application returns the primary product for the product code entered.
 Source: fda.report
Source: fda.report
Antiseptic Hand sanitizers are OTC drugs which require FDA establishment registration Drug listing and NDC Labeler code also known as NDC Number. FDA Regulations for Hand Sanitizers. 25113 - Dodge 25113-444 - Instant Hand Sanitizer 25113-444-15. Registered drug establishments must list the drug eg. FDA approval for hand sanitizer FDA approval is not required for OTC hand sanitizers.
 Source: prevasiveproducts.com
Source: prevasiveproducts.com
By building upon the code portions you select the application will provide valid choices for each of the five components of the product code Industry Class Subclass PIC and Product. 355 are in effect for your PURELL Healthcare Advanced Hand Sanitizers nor are we aware of any adequate and well. So manufacturing import or distribution is permitted only after FDA Hand Sanitizer Registration and Listing. The sanitizer products must be prepared under sanitary conditions that have appropriate equipments in place. After the product is submitted and get approved manufacturers and brand owners can export their products to the US.
 Source: natomask.com
Source: natomask.com
The sanitizer products must be prepared under sanitary conditions that have appropriate equipments in place. While not a drug in the traditional sense the Food and Drug Administration FDA does classify hand sanitizers as an over the counter OTC drug product. FDA approval for hand sanitizer FDA approval is not required for OTC hand sanitizers. So manufacturing import or distribution is permitted only after FDA Hand Sanitizer Registration and Listing. FDA Hand Sanitizer Labeling Requirements.
 Source: indiamart.com
Source: indiamart.com
The FDAs guidance documents apply only to handrub products prepared using the United States Pharmacopoeia or Food Chemical Codex grade ingredients specifically described in the guidance consistent with World Health Organization recommendations from Coronavirus COVID-19 Update. 25113 - Dodge 25113-444 - Instant Hand Sanitizer 25113-444-15. FDA approval for hand sanitizer FDA approval is not required for OTC hand sanitizers. If the product code is valid the name of the product will appear on the next screen. On 24th April 2019 FDA published final rule stating that the three active ingredients namely benzalkonium chloride ethyl alcohol and isopropyl alcoholare the only.
 Source: fdalisting.com
Source: fdalisting.com
Option 4 - Verify Product Code. 355 are in effect for your PURELL Healthcare Advanced Hand Sanitizers nor are we aware of any adequate and well. No FDA approved applications pursuant to section 505 of the Act 21 USC. Amid the novel coronavirus outbreak COVID-19 we are getting many questions about FDA requirements for hand sanitizers and other questions related to FDA regulations for hand sanitizers. 25113 - Dodge 25113-444 - Instant Hand Sanitizer 25113-444-15.
 Source: fda.report
Source: fda.report
While not a drug in the traditional sense the Food and Drug Administration FDA does classify hand sanitizers as an over the counter OTC drug product. Each listed drug is assigned a National Drug Code NDC. If you are planning to market hand sanitizers or any form of antiseptic products we can help you in fulfilling FDA. FDA Hand Sanitizer Registration Approval - I3CGLOBAL Hand Sanitizer FDA Registration Approval Hand Sanitizer is considered as the counter drug OTC as per US FDA regulation. The non-proprietary name is sometimes called the generic name.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title hand sanitizer fda product code by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






